Adhesion to Metal in Modern Manufacturing
In almost every industrial sector, the question arises: how can metals be joined securely and durably without relying solely on screws, rivets, or welding? Adhesion to metal is at the heart of this challenge. While metals naturally offer high surface energy and therefore good conditions for bonding, real-world obstacles such as oxides, lubricants, or coatings often stand in the way of reliable adhesion. Choosing the right pretreatment and adhesive technology is therefore a decisive factor for production efficiency, product quality, and long-term stability.
The relevance spans across industries: in the automotive sector, adhesives enable lightweight construction by joining aluminum or mixed materials without adding excess weight. In aviation, bonding technology reduces drilling points and stress concentrations in thin-walled structures. In electronics and e-mobility, reliable adhesion ensures the performance of sensors, batteries, and housings under demanding thermal and mechanical conditions. Even in medical technology, residue-free and biocompatible adhesion solutions play a central role in guaranteeing safety and functionality.
Strong adhesion to metals is thus more than a technical detail, it is a key enabler of innovation and competitiveness in modern manufacturing.
Technical Basis of Adhesion to Metals
Adhesion describes the attraction between two different materials at their interface, while cohesion refers to the internal strength within a material itself. For reliable bonding to metal surfaces, both must work together: the adhesive must stick to the substrate (adhesion) and withstand internal stresses (cohesion).
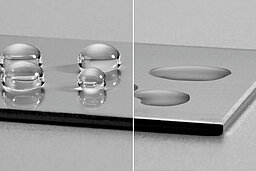
Different mechanisms contribute to adhesion. Mechanical adhesion occurs when an adhesive penetrates surface irregularities, creating a form-lock. Chemical adhesion is based on covalent or ionic bonds between the adhesive and the substrate, which often provides the strongest and most durable connection. Physical adhesion relies on weaker intermolecular forces such as van der Waals interactions, which may still play a role in stabilizing the bond.
A decisive factor for bonding to metals is their high surface free energy. Compared to plastics or composites, metals naturally allow liquids to spread well, provided the surface is clean. This wettability can be quantified by the contact angle: the lower the angle, the better the adhesive wets the surface and forms an intimate contact. The presence of oxide layers—which form spontaneously on metals like aluminum, copper, and magnesium—significantly influences adhesion. These layers can either support bonding by creating polar, chemically active sites or weaken it when they are loosely bound and prone to delamination.
Different metals present different adhesion challenges:
- Aluminum: bonds primarily to its oxide layer, which is not always stable; mechanical or plasma pretreatment improves reliability.
- Stainless steel: usually bonds well, but mirror-polished surfaces may have too low surface energy and require roughening.
- Mild steel: prone to corrosion; surface prep and protective bonding are essential.
- Copper and copper alloys: highly reactive surfaces that oxidize quickly, demanding proper cleaning and fast processing.
In practice, understanding these fundamentals—surface energy, wettability, oxide behavior, and the adhesive mechanisms —is key to selecting suitable adhesives and pretreatments for strong and durable metal bonding.
Surface Pretreatment for Strong Metal Bonds: Clean, Roughen, Activate

A strong and durable bond to metal depends less on the adhesive itself than on the condition of the substrate. Even though metals generally exhibit high surface free energy, contamination, oxides, or coatings can drastically reduce wettability and adhesion. For this reason, surface pretreatment, like cleaning, roughening, and activation, is often the decisive step for reliable bonding.
Surface preparation usually begins with the removal of oils, greases, and lubricants using solvents or alkaline cleaners. On aluminum, for example, the natural oxide layer is unstable and should be removed or stabilized by abrasion, etching, or anodization. Roughening methods such as grinding or sandblasting increase the effective surface area and create micro-retentive structures for mechanical adhesion. Chemical pretreatments like acid etching or alkaline cleaning enhance surface polarity and remove contaminants at the microscopic level.
An increasingly important method is plasma technology. Plasma treatment activates metal surfaces without mechanical contact, modifying the uppermost nanometers of the material. This results in a sharp increase in surface energy, improved wettability, and a clean, selective, and environmentally friendly activation process without solvents or aggressive chemicals. For industries with high process reliability requirements, plasma pretreatment is valued as a sustainable alternative to traditional cleaning methods.
Comparison: Mechanical and chemical pretreatments are well-established and highly effective but require labor, consumables, and in many cases generate waste. Plasma activation, by contrast, is cleaner and more controllable, though it requires investment in equipment. In practice, companies often combine methods (e.g., solvent cleaning + plasma activation) to ensure reproducible adhesion.
Common Adhesion Failures Explained
Despite high theoretical surface energy, in practice metals often fail to bond as expected. The main reason is residues (machining oils, release agents, or fingerprints) that act as barriers to wetting. Insufficient cleaning or inadequate choice of pretreatment can leave weak oxide layers, especially on aluminum, which later delaminate. Coated or painted metals present another challenge, since the adhesive bonds to the coating, not the base metal, meaning the coating’s adhesion becomes the limiting factor.
A further risk is galvanic corrosion, which occurs when adhesives are used incorrectly between dissimilar metals without considering electrochemical compatibility. This can lead to unexpected failure even if the initial bond strength is high. Finally, a frequent misconception lies in the belief in “all-purpose adhesives”. While some products are marketed as universally applicable, reliable industrial bonding demands tailored adhesive systems combined with the right pretreatment strategy.
Plasma Activation of Metal Surfaces for Stronger Bonds
Plasma treatment is one of the most effective technologies for improving adhesion to metals. In contrast to purely mechanical or chemical processes, plasma acts on the very top atomic layers of the substrate, modifying its surface without affecting the bulk properties. Three effects can be distinguished: cleaning (removal of organic residues and thin films), activation (increasing surface energy for improved wettability), and functionalization (introducing chemical groups that enable stronger interaction with adhesives and coatings).
The key advantage lies in its precision and selectivity. Plasma treatment can target only the bonding area, leaving other functional areas untouched. Since the process is contact-free and operates at low temperatures, the metal surface is not mechanically damaged or overheated. Compared to solvent cleaning or aggressive chemical etching, plasma is environmentally friendly, reproducible, and safer, as it eliminates the use of hazardous chemicals and minimizes waste streams.
Industrial Uses of Plasma Adhesion

- Automotive industry: Plasma activation of aluminum or steel parts before adhesive joining or coating improves bonding strength and corrosion resistance, particularly in lightweight construction.
- Medical technology: Plasma cleaning ensures residue-free, high-energy surfaces for biocompatible coatings or joining stainless steel instruments without introducing contaminants.
- E-mobility and electronics: Plasma pretreatment of copper or aluminum improves insulation and bonding in battery housings, sensors, or electronic assemblies, where reliability under thermal and mechanical loads is critical.
From a production perspective, plasma systems can be integrated directly into existing manufacturing lines. Inline plasma units treat parts immediately before bonding or coating, ensuring high process stability and eliminating delays between pretreatment and adhesive application. This makes plasma not only a technical enabler of stronger adhesion, but also a tool for leaner, more sustainable manufacturing processes.
Adhesive Types Compared for Metals

Choosing the right adhesive for metal bonding depends on the application environment, load profile, and processing requirements. Different adhesive classes offer distinct advantages and limitations, making selection a critical step in achieving durable performance.
Epoxy adhesives are among the most widely used for metals. They provide high tensile and shear strength, excellent temperature resistance, and superior chemical and environmental durability. Epoxies are therefore ideal in aerospace, automotive, and industrial machinery, where reliability under heat and aggressive media is essential. Their main drawback is longer curing times and lower flexibility compared to other systems.
Acrylic adhesives, especially structural methacrylates (MMA), are valued for their fast curing, high impact resistance, and tolerance to less-than-perfect surface preparation. In laboratory comparisons, they often outperform other classes when joining coated or galvanized metals. Their balance of speed, toughness, and versatility makes them attractive for transportation and construction, though odor and exotherm during curing can be limiting factors.
Cyanoacrylates (instant adhesives) bond metals within seconds and are ideal for small parts, precision assembly, or quick fixturing. However, their durability under long-term stress, humidity, or high temperatures is limited. They are best reserved for secondary bonds or components not exposed to harsh conditions.
Polyurethane adhesives combine flexibility with strong adhesion, making them well-suited to applications with vibration, dynamic loads, or thermal expansion. Their inherent moisture resistance adds value in outdoor or humid environments. The trade-off is lower strength and heat resistance compared to epoxies or structural acrylics.
Beyond adhesive chemistry, selection criteria should include:
- Type of load (shear, peel, impact, vibration)
- Operating temperature range and exposure to chemicals or humidity
- Differences in thermal expansion between bonded materials
- Processing factors, such as cure time, gap-filling capacity, and production speed
Across all classes, adhesives offer clear advantages over welding, riveting, or screwing: they distribute stress more evenly, eliminate the need for drilling or heating, reduce galvanic corrosion risks, and enable lightweight, streamlined designs. For these reasons, structural adhesives are increasingly used as a primary joining method in industries where strength, durability, and efficiency must be balanced.
Measuring & Documenting Adhesion for Maximum Quality
For industrial applications, ensuring that adhesive bonds are reliable over the long term requires objective measurement and thorough documentation. Since adhesion depends strongly on surface condition, testing and monitoring methods are indispensable for both development and production.
Contact angle measurement is the most common way to verify surface cleanliness and activation. A drop of liquid is placed on the metal surface, and the angle formed at the interface is measured. Low contact angles indicate good wettability and high surface energy, which are prerequisites for strong adhesion. More advanced techniques calculate the surface free energy (SFE) directly, allowing companies to set quantitative thresholds for acceptable pretreatment.
In addition to surface tests, the mechanical strength of the bond is evaluated through standardized procedures:
- Pull tests (tensile tests): measure the maximum force required to separate bonded parts in tension.
- Peel tests: assess how the adhesive joint performs under peeling forces, which are particularly critical for thin or flexible components.
- Shear tests: evaluate load-bearing capacity when forces act parallel to the bond line, simulating many real-world applications.
Equally important is documentation and reproducibility. Establishing clear process parameters, like pretreatment steps, adhesive batch, curing conditions, and linking them with test data ensures traceability and quality control. In modern production, inline monitoring systems can integrate contact angle or pull test data directly into quality management systems, making adhesion a controlled and certifiable process rather than a variable outcome.
FAQ: Common Questions on Metal Bonding
How do I achieve adhesion on stainless steel?
Most stainless steels bond well after degreasing. However, mirror-polished surfaces may have too low surface energy. In this case, roughening (e.g., sandblasting) or plasma activation improves wetting and adhesive strength.
What should I do if the bond comes loose?
Check for contamination, insufficient pretreatment, or environmental overload (temperature, humidity, stress). Reassess the adhesive choice and ensure correct surface cleaning and curing. Weak or unstable oxide layers (e.g., on aluminum) are often the root cause.
How do I find the right plasma device?
Selection depends on part size, throughput, and integration level. For inline production, automated plasma systems are suitable; for R&D or small series, compact benchtop devices offer flexibility. Always consider process monitoring options to guarantee reproducibility.
Which adhesive is best suited for aluminum?
Aluminum forms unstable oxide layers that can undermine adhesion. Epoxies or structural acrylics (MMA) generally deliver the most reliable results, but only when combined with proper pretreatment (abrasion + cleaning or plasma activation).
How can I improve adhesion to metal?
Start with thorough cleaning, remove oxides or coatings where necessary, and use plasma activation for stable surface energy. Then match adhesive type to load, environment, and curing constraints.
Which tests guarantee reliable results?
Contact angle and surface energy measurements verify surface readiness. Pull, peel, and shear tests under standardized conditions (ISO/ASTM) ensure mechanical strength. Documenting these results is key to quality assurance.
How important is surface pretreatment?
Crucial. Even though metals have high surface energy, residues, weak oxides, or coatings can block wetting. Without proper pretreatment, the best adhesive cannot compensate for poor adhesion conditions.
Can adhesives replace welding or riveting in metal construction?
Yes, in many cases. Structural adhesives distribute stress more evenly, prevent heat distortion, and avoid drilling holes. However, joint design and load requirements must be carefully evaluated to ensure long-term durability.
How do I avoid galvanic corrosion when bonding dissimilar metals?
Use adhesives as an isolating layer between the metals. Ensure full coverage of the bonding area and select adhesives resistant to moisture ingress, which can trigger galvanic effects.
What role does curing time play in bond reliability?
Curing time directly affects adhesive performance. Insufficient curing leads to weak bonds and premature failure. Always follow manufacturer specifications for temperature, pressure, and time to achieve full mechanical strength and chemical resistance.
Are universal adhesives suitable for industrial metal bonding?
Generally not. While “all-purpose” adhesives may provide quick fixes, they cannot match the strength, durability, and environmental resistance of specialized structural adhesives tailored for metals.